Abstract
Introduction: To this point, T-cell engager (TCE) bispecific antibodies have been developed for multiple myeloma (MM) from approaches that use exogenous donor T cells or cell lines. This new class of therapy is showing high response rates in end-stage patients with multiple myeloma, with options targeting extracellular antigens including BCMA, GPRC5D, FCRH5 and CD38. Here, we demonstrate that Myeloma Drug Sensitivity Testing (My-DST) can measure efficacy of TCE antibodies ex vivo in primary MM harnessing the patients’ own endogenous immune cells. This allows the study of TCE antibody sensitivity in a setting that can recapitulate the immune exhaustion often observed in heavily pretreated relapsed/refractory myeloma (RRMM) patients. RRMM is aggressive, therapy-resistant and leads to shortened survival for most patients. To address this, maximizing the application of novel immunotherapies will be paramount. One such agent with potential for these patients is CD38/CD28xCD3 trispecific TCE (CD38 TriAb), which showed anti-tumor activity in CD38-dim cell lines (Wu et al, Nature Cancer. 2019). Recently, we found CD38 recovery one year after the last dose of daratumumab (Dara), making patients candidates for re-treatment with anti-CD38 therapy (Perez de Acha et al, ASH 2020). Here, we show the anti-MM cytotoxicity of CD38 TriAb with My-DST in heavily pretreated patients that have relapsed after anti-CD38 and anti-BCMA immunotherapies.
Methods: Bone marrow and peripheral blood samples were collected from MM patients with informed consent and IRB approval. Mononuclear cells (MNCs) were isolated, incubated for 48h with CD38 TriAb, nullCD38, nullCD28, daratumumab, isatuximab, or controls in triplicate wells for 48 h and MM cell survival analyzed by flow cytometry. Samples were stained with either a MM panel containing CD38, CD138, BCMA, CD46, CD56, CD45, CD19 and CD28 or an immune cell panel containing CD3, CD4, CD107a, CD107b, CD28, CD38, CD16, CD8 and CD56 antibodies conjugated to fluorophores. GolgiStop (BD) was added to culture for 2h before harvest per manufacturer instructions to allow measurement of CD107a and CD107b. ANOVA with multiple comparison correction was used for statistical significance.
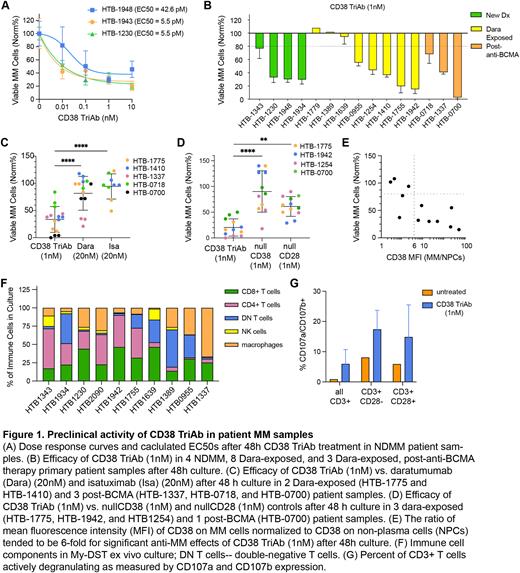
Results: My-DST with CD38-TriAb was performed in samples from 15 patients (4 newly diagnosed [NDMM], 8 Dara-exposed, and 3 relapsed after anti-BCMA). CD38 TriAb titration in NDMM patient samples showed potent picomolar EC50s (Fig 1A). To judge sensitivity vs resistance, the approximate EC90 of 1nM was chosen to screen samples at single concentration doses. MM viability of <80% was used as the threshold of sensitivity, as the at which most tests reach statistical significance. Out of 15 patient samples tested, 11 responded (73.3%) (Fig 1B). Of these, 3 NDMM, 5 Dara-exposed, and 3 post-BCMA patients responded. Compared to anti-CD38 mAbs Dara and Isatuximab (Isa), CD38 TriAb displayed significantly higher efficacy than both mAbs (Fig 1C). CD38 TriAb also had significantly better efficacy compared to controls lacking the anti-CD28 arm (Fig 1D). Notably, all patients who failed to respond to CD38 TriAb had dim CD38 expression. In most cases, CD38 expression was at least sixfold higher on MM than surrounding MNCs for patient samples that responded to CD38 TriAb (Fig 1E), a similar threshold has been observed by our lab with Dara-treated. Diverse immune cell populations were present in the samples evaluated, including helper and cytotoxic T cells (Fig 1F). CD38 TriAb also successfully induced degranulation of CD3+CD28- and CD3+CD28+ cytotoxic T cells (Fig 1G).
Conclusion: The above data leads us to conclude that (1) CD38 TriAb is extremely potent, displaying EC50s in the picomolar range and (2) My-DST is able to measure CD38 TriAb efficacy ex vivo in primary samples through the patients’ endogenous T cells to more closely recapitulate clinical administration. Further analysis is required to confirm that this data predicts a high response rate to CD38 TriAb in patients relapsed after anti-CD38 and anti-BCMA therapy. My-DST may be more broadly applicable to personalizing TCE treatment decisions, as individual patients may benefit from targeting one antigen over another in relapsed refractory multiple myeloma.
Disclosures
Keller:Sanofi: Consultancy, Research Funding. Perez De Acha:Sanofi: Consultancy, Research Funding. Reiman:Sanofi: Consultancy, Research Funding. Walker:Sanofi: Consultancy, Research Funding. Niesvizky:Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Forsberg:University of Colorado: Current Employment; BMS, GSK: Consultancy; Karyopharm: Research Funding. Kim:Sanofi: Current Employment, Other: May hold shares and/or stock options in the company. Bisht:Sanofi: Consultancy, Current Employment, Other: May hold shares and/or stock options in the company. Wang:Sanofi: Current Employment, Other: May hold shares and/or stock options in the company. van de Velde:Sanofi: Current Employment, Other: May hold shares and/or stock options in the company. Sherbenou:Sanofi: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal